Previous studies have established that RG-II together with RG-I and a mixture of oligogalacturonides are solubilized by EPG treatment of flowering plant walls.
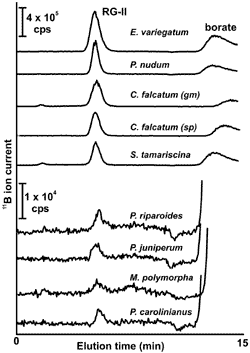
The material solubilized by EPG was fractionated by size-exclusion chromatography (SEC) using a Superdex-75 HR10/30 column and refractive index detection (see figure on right). Peaks that elute in the region for the borate cross linked RG-II dimer and the RG-II monomer are clearly present.
No peaks corresponding to the RG-II monomer or dimer were detected when the material solubilized by EPG treatment of the bryophyte walls was analyzed by SEC-RI.
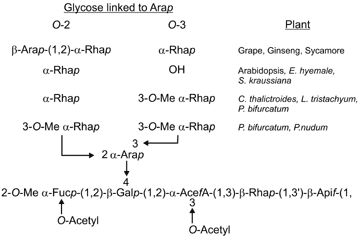
The material in the peaks corresponding to RG-II from several different lycophytes and pteridophytes was collected and analyzed in detail.
Glycosyl residue and glycosyl-linkage composition analyses, together with 11B-NMR spectroscopy, confirmed that RG-II was present in these walls as a borate cross linked dimer.
It is clear from our results (1) that RG-II is indeed present in the walls of non-flowering vascular plants that include lycophytes, psilopsids, horsetails and ferns.