 |
|||||||
 |
|||||||
| Direct Coupling of Glycans to Protein Oligo- and polysaccharides have a hemiacetal or hemiketal function at their reducing end. The cyclic form of the reducing sugar (1) is in equilibrium with the open chain aldehyde (2). This equilibrium favors the cyclic hemiacetal (1). Primary amines react reversibly with free carbonyls to form an imine (Schiff base, 3) that is converted to a stable secondary amine (4) by reduction. |
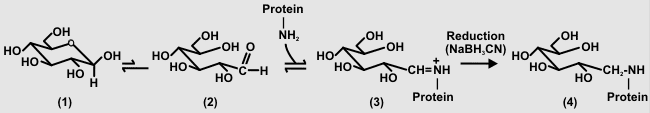 |
The overall rate of this reaction is dependent on the slow formation of the imine (3). Typically, the reaction between the glycan is allowed to proceed at pH 8 for between 5 and 10 days. Nevertheless, this simple procedure performed under mild conditions is likely to have minimal affects on the glycan. We will perform the coupling reaction with "Super Carrier" (Pierce Chemical Co). Super Carrier is a modified bovine serum albumin whose carboxyl groups have been converted to primary amines. In our experience "Super Carrier" gives a 10-fold increase in serum titers and a increased yield of anti-glycan antibody secreting hybridoma lines. |
Formation and Coupling of Activated Glycans to Protein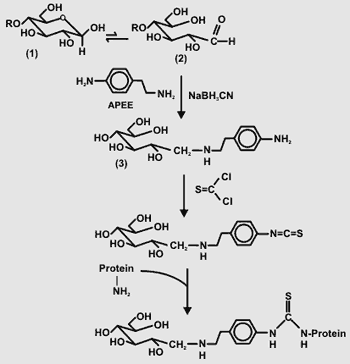 Polysaccharides have several functional groups (carbonyl, carboxyl, and hydroxyl) that can be activated and then used to couple the glycan to "Supere Carrier". Polysaccharides have several functional groups (carbonyl, carboxyl, and hydroxyl) that can be activated and then used to couple the glycan to "Supere Carrier".We have succesfully used the procedure developed by Smith and Ginsburg (J. Biol. Chem. [1980] 255, 55-59) in which the reducing glycose is reacted with 2-(4-aminophenyl)ethylamine (APEE) in the preseence of sodium cyanoborohydride (see Figure on right). The primary amine is then reacted with thiophosgene to generate the phenylisothiocyanate intermediate which itself can be coupled to "Super Carrier". We have generated several neoglycoconjugate antigens using this procedure. However, there are several potential problems. The glycan must be soluble in APEE. The formation of the imine is relatively slow. Thiophosgene is a reactive (and toxic) reagent. Thus, the possibility cannot be discounted that side reactions that affect the structure of the glycan occur during the formation of the isothiocyanate. The glycan is linked to the protein by a aromatic residue in the neo-glyconjugate. Aromatic linkers are often immunodominant. |
Coupling of SH-Containing Glycans to Maleimide-Activated Protein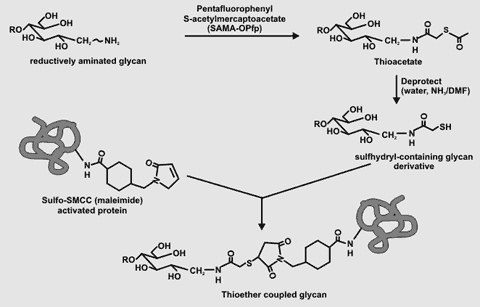 Reacting a protein with sulfosuccinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate
(Sulfo-SMCC) results in the formation of an maleimide activated protein.
This protein can then be reacted with compounds that contain a free sulfhydryl
group to form a stable thioether bond. Several maleimide activated proteins
(e.g. BSA and keyhole limpet hemocyanin) are commercially available Reacting a protein with sulfosuccinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate
(Sulfo-SMCC) results in the formation of an maleimide activated protein.
This protein can then be reacted with compounds that contain a free sulfhydryl
group to form a stable thioether bond. Several maleimide activated proteins
(e.g. BSA and keyhole limpet hemocyanin) are commercially available Oligosaccharides with a sulfhydryl group at their former reducing end can be synthesized. For example, the amino group of a reductively aminated glycan can be converted to the thioacetate derivative by reaction with SAMA-OPfp (see figure on right). Deprotection of the thioacetate results in the formation of the SH-containing glycan. The SH-containing glycan is rapidly (~2h reaction time) coupled to a maleimide activated protein at pH 7.2 A disadvantage of this procedure is that the cross-linker contains a hindered ring structure that is itself highly immunogenic. This problem can be minimized by the use of proteins acivated with aliphatic straight chain spacers (e.g. N-(4-maleimidobutyryloxy) sulfosuccinimide ester "Sulfo-GMBS") that exhibit low immunogenicity. Back to Top |
|
|
| [Home] | [People] | [Background] | [Research Plan] | [Wall Antigens] | [Antibodies] | [Facilities] | [Links] |
|
|
| A National Science Foundation-funded (Grant No DBI-0421683) research project at The Complex Carbohydrate Research Center of The University of Georgia |