 |
 |
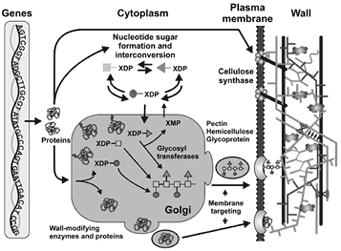 |
|
| Overview |
| Plant cell wall research at the CCRC is carried out by six independently
funded groups. These groups study diverse topics that include the primary
structure and three-dimensional conformations of wall components, the interactions
of wall components, and the biosynthesis of wall components. In addition
we are studying the role of the cell wall as a source of biological active
molecules, as a barrier to plant pathogens, and as a source of biofuels. Click on a link to obtain information on: |
|
What is a primary cell wall |
Techniques used at the CCRC to study walls Selected References |
|
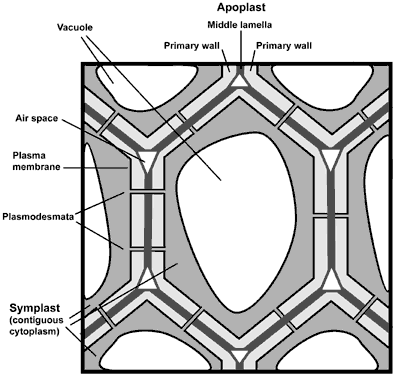
Growing plant cells are surrounded by a polysaccharide-rich primary
wall. This wall is part of the apoplast which itself is largely self-contiguous
and contains everything that is located between the plasma membrane and
the cuticle. The primary wall and middle lamella account for most of the
apoplast in growing tissue. The symplast is another unique feature of plant
tissues. This self-contiguous phase exists because tube-like structrues
known as plasmodesmata connect the cytoplasm of different cells. |
 |
|
Some of the functions of the primary wall:
|
Primary walls are the major textural component of plant-derived foods. The ripening of fruits and vegetables is associated with changes in wall structrue and composition. Plant-derived beverages often contain significant amounts of wall polysaccharides. Some wall polysaccharides bind heavy metals, stimulate the immune system or regulate serum cholesterol. Wall polysaccharides are used commercially as gums. gels, and stabilizers. Thus, cell wall structure and organization is of interest to the plant scientist, the food processing industry and the nutritionist. |
|
Primary wall composition and architecture The major polysaccharides in the primary wall are: |
|
The organization and interactions of wall components is not known with certainty and there is still considerable debate about how wall organization is modified to allow cells to expand and grow. Several models have been proposed to account for the mechanical properties of the wall: |
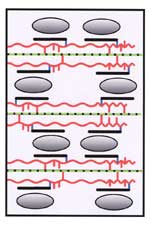
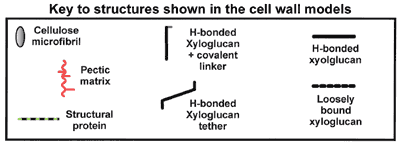
| The covalently cross-linked model Peter Albersheim and colleagues in 1973 proposed that the wall matrix polymers (xyloglucan, pectin, and glycoprotein) are covalently linked to one another. The binding of xyloglucan to cellulose microfibrils results in a non-covalently cross-linked cellulose-hemicellulose network that gives the wall tensile strength. This model has been questioned because of the lack of evidence for the existence of covalent linkages between xyloglucan, pectin and glycoprotein. |
 |
 |
| The tether model |
The diffuse layer model |
The stratified layer model |
Xyloglucan molecules are hydrogen bonded to and cross-link cellulose microfibrils. The cellulose-xyloglucan network is emeshed in a non-covalently cross-linked pectic network.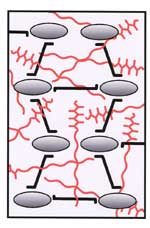 |
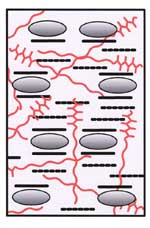 Xyloglucan molecules are hydrogen bonded to the surface of cellulose microfibrils but do not directly cross link them. The tightly-bound xyloglucan is surrounded by a layer of less-tightly bound polysaccharides. The cellulose and xyloglucan are embedded in a pectic matrix. |
Xyloglucan molecules are hydrogen bonded to and cross-link cellulose microfibrils. The cellulose-xyloglucan lamellae are separated by strata of pectic polysaccharides.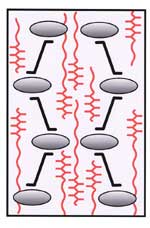 |
Much research is sitll required to provide a complete description of the primary wall at the molecular level. Moreover, there is increasing evidence that primary walls are dynamic structures whose composition and architecture changes during plant growth and development.
|
|
Plants form two types of cell wall that differ in function and in composition. Primary walls surround growing and dividing plant cells. These walls provide mechanical strength but must also expand to allow the cell to grow and divide. The much thicker and stronger secondary wall (see figure on right), which accounts for most of the carbohydrate in biomass, is deposited once the cell has ceased to grow. The secondary walls of xylem fibers, tracheids, and sclereids are further strengthened by the incorporation of lignin. The evolution of conducting tissues with rigid secondary cell walls was a critical adaptive event in the history of land plants, as it facilitated the transport of water and nutrients and allowed extensive upright growth. Secondary walls also have a major impact on human life, as they are a major component of wood and are a source of nutrition for livestock. In addition, secondary walls may help to reduce our dependence on petroleum, as they account for the bulk of renewable biomass that can be converted to fuel. Nevertheless, numerous technical challenges must be overcome to enable the efficient utilization of secondary walls for energy production and for agriculture. |
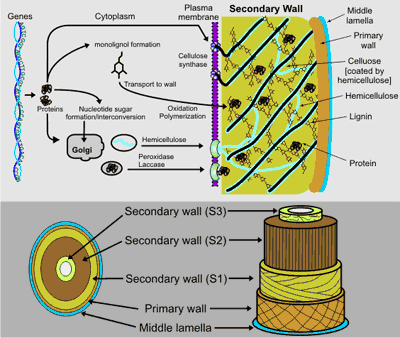 |
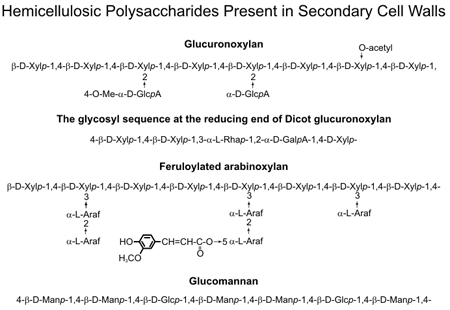
Primary and secondary walls contain cellulose, hemicellulose and pectin, albeit in different proportions. Approximately equal amounts of pectin and hemicellulose are present in dicot primary walls whereas hemicellulose is more abundant in grasses (e.g., switchgrass). The secondary walls of woody tissue and grasses are composed predominantly of cellulose, lignin, and hemicellulose (xylan, glucuronoxylan, arabinoxylan, or glucomannan). The cellulose fibrils are embedded in a network of hemicellulose and lignin. Cross-linking of this network is believed to result in the elimination of water from the wall and the formation of a hydrophobic composite that limits accessibility of hydrolytic enzymes and is a major contributor to the structural characterisitics of secondary walls. Xylan, which accounts for up to 30% of the mass of the secondary walls in wood and grasses contributes to the recalcitrance of these walls to enzymic degradation. A high xylan content in wood pulp increases the economic and environmental costs of bleaching in paper manufacturing. Thus, reducing the xylan content of secondary walls and altering xylan structure, molecular weight, ease of extractability, and susceptibility to enzymic fragmentation are key targets for the genetic improvement of plants. However, progress in these areas is limited by our incomplete understanding of the mechanisms of xylan biosynthesis. |
Xylans have a backbone of 1,4-linked β-D-xylosyl residues with short [α-D-glucosyluronic acid (GlcA), 4-O-methyl-α-D-glucosyluronic acid (MeGlcA), α-L-arabinosyl, O-acetyl, feruloyl, or coumaroyl] sidechains (see figure). Xylan synthesis requires the coordinated action of numerous enzymes, including glycosyl transferases (GTs) that elongate the backbone and add side chain residues (see left figure on right). None of these GTs have been purified and biochemically characterized, although several candidate genes have been identified. Little is known about the factors that regulate secondary wall polysaccharide biosynthesis and the mechanisms that control the assembly of these polysaccharides into a functional wall. Moreover, the mechanisms required to initiate and terminate xylan synthesis and to control the chain length of the xylan are as yet unidentified. |
 |
|
Techniques used at the CCRC to study walls We are interested in several aspects of primary and secondary walls including:
|
| Plants | Cultured Cells | ||
| Arabidopsis | Arabidopsis | ||
| Tomato | Tomato | ||
| Tobacco | Sycamore | ||
| Lemna | Rice | ||
| Ferns | Soybean | ||
| Horsetails | Grapefruit | ||
| Lycopods | Physcomitrella (moss) | ||
| Mosses | Anthoceros (hornwort) | ||
| Liverworts | Chara (green algae) | ||
| Hornworts | Coleochaete (green algae) |
|
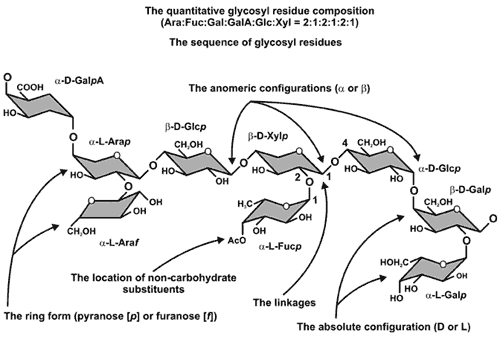
The primary structures of wall polysaccharides |
 |
|
The conformation and interaction of wall polysaccharides The biosynthesis of pectic polysaccharides |
Selected references Ebringerova (2006) Structural diversity and application potential of hemicelluloses. Macromol Symp 232, 1-12. Ragauskus et al (2006) The path forward for biofuels and biomaterials. Science 311, 484-489. |