 |
 |
| Background |
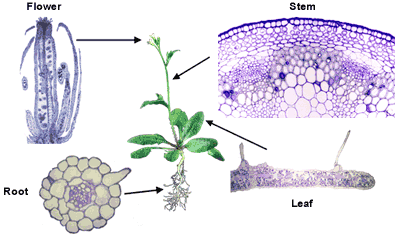
| The diversity in shape and size of flowering plants results from the different
morphologies of the various cell types that make up the vegetative and
reproductive organs of the plant body. These cell types may vary in form
and often have specialized functions. The variety of cell types in a dicotyledenous plant is show in the images of the cross sections of the major organs (flower, stem, leaf, and root) of Arabidopsis. If you click on a image you will see a larger image. WARNING - these images are quite large (~40K). |
 |
Changes in tissue and organ morphology that occur during plant growth and development result in large part from controlled cell division together with the structural modification and reorganization of wall components, and the synthesis and insertion of new material into the existing wall. Nevertheless, the biochemical and physical factors that regulate wall modification and expansion are not fully understood. |
| A goal of our research is to understand how the structures of cell wall
components and their organization change during plant growth and development
and during a plants interaction with other organisms and its environment.
This type of information can be obtained by the developemnt and application
of molecular probes that can be to locate individual macromolecules. Thus,
we are generating monoclonal antibodies to use as probes to study cell
wall structure and dynamics. In August 2004 we were awarded a NSF grant (Grant number DBI-0421683) to generate a library of monoclonal antibodies that recognize carbhydrate structures within the plant cell wall. Additional information is available at the Monoclonal Antibodies for Plant Cell Walls web site |
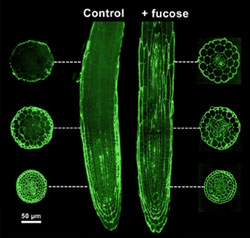
| Our research has focused on the distribution in plant cell walls of carbohydrate
epitopes recognized by several monoclonal antibodies. Studies of the Arabidopsis mur1 mutant, using CCRC-M1, have provided evidence for the existence of domains within the walls of a single cell. CCRC-M1 recognizes a fucosyl-containing epitope of xyloglucan. The figure on the right shows the distribution of the fucose epitope in a root of a mur1 seedling using immunoflorescence labeling. Only specific cells of the mur1 roots are capable of synthesizing GDP-fucose. The M1 epitope is adundant in all cells at the root tip. The intensity of labeling diminishes along the length of the root. The M1 epitope is distributed throughout the root when mur1 plants are grown in the presence of fucose. |
 |
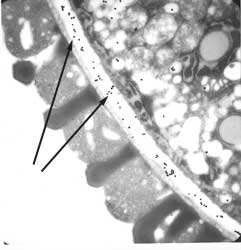
| In collaboration with Wolf-Dieter Reiter (U. Connecticut) we have shown,
using immunocytochemical and genetic methods, that the two biosynthetic
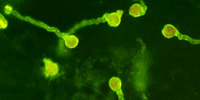
pathways for fucose biosynthesis in Arabidopsis are alternatively utilized in different cell types and at diferent times. The figure on the right shows that the epitope recognized by CCRC-M1 is present in the pollen cell walls of the Arabidopsis mur1 mutant. The fucose epiotpe is revealed by immunogold-labeling of the pollen (arrows). The figure below shows CCRC-M1 labeling (FITC immunofluorescent) of germinating mur1 pollen: |
 |
 |
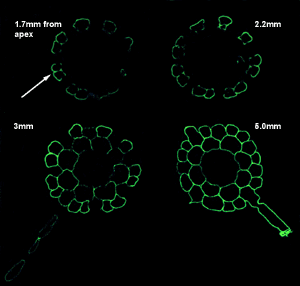
| The insertion of the carbohydrate epitope in RG-I recognized by the monoclonal antibody CCRC-M2 is associated with differentiation of epidermal and cortical cells. | |
In immature Arabidopsis roots (<2.2mm from the apex), CCRC-M2 labels only those epidermal cells (see arrow) that do not form root hairs. The root hair-forming epidermal cells and the interior cell layers within the root do not label. In mature roots (>3mm from the apex) CCRC-M2 labels all epidermal and cortical cells but not endodermal, xylem, or phloem cells. |
 |
| Current research 1. Generation of monoclonal antibodies against RG-II. 2. Characterization of the epitopes recognized by CCRC-M2. 3. The use of monoclonal antibodies to characterize the cell walls of lycophytes and pteridophytes. These immunocytochemical methods will complement our structural analyses of spore-bearing tracheophytes walls using chemical and enzymic methods. |
| Selected references Puhlmann et al (1994) Generation of monoclonal antibodies against plant cell wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal α-(1->2)-linked fucosyl-containing epitope. Plant Physiol., 104, 699-710. Steffan et al (1995). Characterization of a monoclonal antibody that recognizes an arabinosylated (1->6)-β-D-galactan epitope in plant complex carbohydrates. Carbohydr. Res., 275, 295-307 Freshour et al (1996) Developmental and tissue-specific structural alterations of the cell wall polysaccharides of Arabidopsis thaliana roots. Plant Physiol., 110, 1413-1429. Williams et al (1996). An antibody Fab selected from a recombinant phage display library detects deesterified pectic polysaccharide rhamnogalacturonan II in plant cells. Plant Cell, 8, 673-685. Freshour et al (2003) Distribution of fucose-containing xyloglucans in cell walls of the mur1 mutant of Arabidopsis. Plant Physiol., 131, 1602-1612. Bonin et al (2003) The GMD1 and GMD2 genes of Arabidopsis encode isoforms of GDP-mannose-4,6-dehydratase with cell type- specific expression patterns. Plant Physiol., 132, 883-892. Zhong et al (2003) Expression of a mutant form of cellulose synthase AtCesA7 causes dominant negative effect on cellulose biosynthesis. Plant Physiol., 132, 786-795. |