 |
 |
| Background |
|
Rhamnogalacturonans (RGs) are a group of closely related cell wall pectic
polysaccharides that contain a backbone of the repeating disaccharide: The term rhamnogalacturonan I (RG-I) is typically used to refer to this pectic polysaccharide |
|
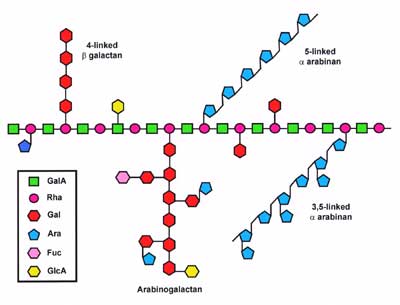
Between 20 and 80% of the Rhap residues are, depending on the plant source and the method of isolation, substituted at C-4 with neutral and acidic oligosaccharides. The oligosaccharides contain linear and branched α-L-Araf and β-D-Galp residues. Some of these side chains may be terminated with α-L-Fucp, β-D-GlcpA, and 4-O-methyl β-D-GlcpA residues (see right figure). The backbone GalpA is not typically substituted with oligosaccharides, although this residue
has been reported to be substituted with a β-D-GlcpA in sugar beet RGs. In contrast the backbone GalpA residues are O-acetylated in RGs from many plants. |
 |
|
Little is know about the function of RGs (2, 3). However, there is increasing evidence that changes in the structures of the arabinan and galactan side chains are correlated with different stages of cell and tissue development. Studies with transgenic plants containing endoglycanses that fragment the RG backbone and side chains have revelead that altering RG structure may have significant affects on plant growth and development (4). |
Current research A covalent linkage between pectin and xyloglucan was a feature of the cell wall model proposed by Albersheim and co-workers in 1973 (Keegstra et al Plant Physiol 51, 188-196). Numerous studies have shown that a portion of the xyloglucan solubilized with 4M KOH binds to anion-exchange resins (Thompson and Fry [2000] Planta 211, 275-286; Vidal et al [2003] Carbohydr Polymers 54, 439-447; Popper and Fry [2005] Anals Botany]. These results together with enzymic fragmentation studies of putative pectin-hemicellulose complexes (Femenia et al [1999] Carbohydr Polymers 39, 151-164; Abdel-Massih et al [2003] Planta 216, 503-511) have provided additional support for an assocaition between hemicellulose and pectin. However, no direct evidence for a covalent linkage has been obtained. Moreover if such a linkage does exist it is not known if hemicellulose is linked to pectin or if pectin is linked to hemicellulose. We have isolated large quantitites of a "anionic xyloglucan" from Arabidopsis leaf cell walls and are now using various endo-glycanses to generate fragments that may contain the hemicellulose-pectin linkage region. These fragments will be characterized using glycosyl composition and linkage analyses, electrospray mass spectrometry and NMR spectroscopy. |
Selective Chemical Fragmentation of rhamnogalacturonans We have developed chemical methods to selectively methyl-esterify and then cleave 4-linked GalA residues in pectic polysaccharides (5). The carboxyl groups of the GalA residues are selectively methyl-esterified using iodomethane in DMSO containing 8% water and tetrabutylammonium fluroide (TBAF). The methyl-esterified pectin is then fragmented by β-elimination in 0.2 M sodium tetraborate, pH 7.3, at 125C.
The oligoglycosyl fragments, which contain a non-reducing 4-deoxy-β-L-threo-hex-4-enepyranosyluronic acid, were characterized using 1D and 2D NMR spectroscopy, MALDI-TOF-MS, and glycosyl-residue composition analyses. Application of this method to a branched rhamnogalacturonan from potato generated fragments that contained two backbone residues and a monogalactosyl side chain. By contrast, fragments containing a minimum of four backbone residues are generated by treating potato RG with a fungal RG lyase. Our chemical method facilitates the release and structural characterization of the oligoglycosyl sidechains of rhamnogalacturonans and also provides a convenient method for generating fully or partially methyl-esterified pectins. We are continuing to refine this methodolgy and are currently developing methods to: 1. Simultaneously β-eliminate the GalA residue and fluorescently label the released reducing glycose. 2. Sequencing RGs using chemical β-elimination together with enzymic fragmentation. |
Selected references (5) Deng, C et al (2006) Selective chemical depolymerization of rhamnogalacturonans. Carbohydr. Res., 341, 474-484. |