 |
 |
|
Rhamnogalacturonan II - structure and properties Rhamnogalacturonan (RG-II) was first described in 1978 (1) and its structure has subsequently been shown to be conserved in the primary walls of dicotyledenous and monocotyledenous plants and gymnosperms. RG-II is also likely to be present in the walls of some lower plants (ferns, horsetails, and lycopods). |
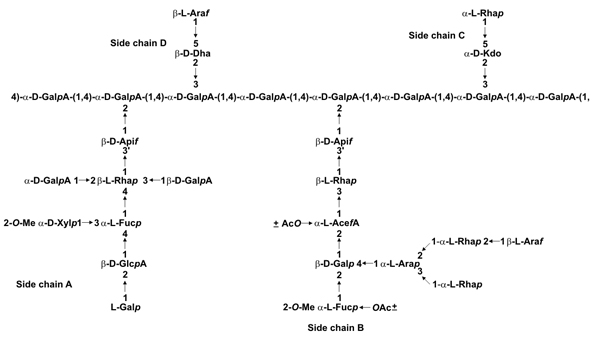 |
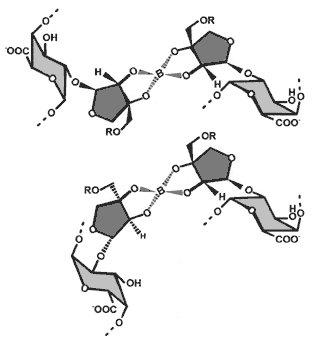
| RG-II exists as a borate cross-linked dimer For many years RG-II remained a enigmatic polysaccharide with no known function. However, in 1996 Matoh and colleagues demonstrated conclusively that RG-II exists in the primary wall as a dimer that is cross linked by a 1:2 borate-diol diester (4). Subsequently, research at the CCRC showed that the dimer contains a single borate diester cross link and that the ester is formed between the apiosyl residue in side chain A of each monomer subunit (5, 6). The borate atom is chiral and thus two diasteroisomers can form (see figure on the right). It is not known which of the two isomers is present in naturally occuring RG-II (7) |
 |
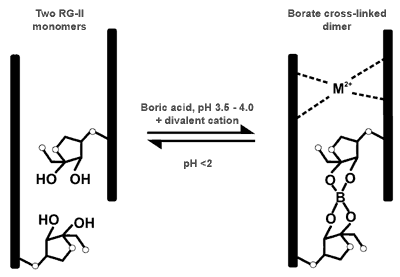
| Formation of the borate ester cross link Research at the CCRC has shown that the monomer and dimer are readily interconverted in vitro. The dimer is converted to the monomer at low pH (pH 1 for 30 min). The monomer is partially converted to the dimer when reacted with boric acid at pH 3.5 for 24 h. Divalent cations (e.g. Sr, Pb, and Ba) with ionic radii >1.0A dramatically increase the rate of this reaction - 95% of the monomer is converted to the dimer in 30 - 60 min (5, 6). The cation is likely to stabilize the cross-link because the ester is partially cleaved by calcium chelators (11, 12). There is evidence that in muro calcium stabilize the borate cross link (11, 12). |
 |
|
Little is known about how the structural complexity of RG-II contributes
to its biological function, although some clues have begun to emerge. For
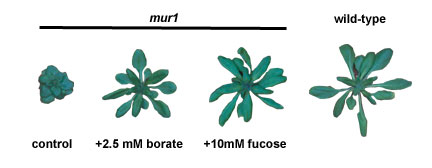
example, the Arabidopsis mur1 mutant synthesizes RG-II that contains L-Gal rather than L-fuc residues. The mur1 RG-II dimer forms less rapidly and is less stable than the normal dimer (13). |
|
Borate cross linking of RG-II and the pectic network of primary cell walls A relationship between boron and wall pectin was first suggested in 1933. Subsequent studies have confirmed that a plants' B requirement and wall pectin content are correlated (8). Plants whose walls contain little pectin (e.g. the grasses) have a much lower B requirement than dicots and non-graminaceous monocots. Several reseachers have proposed that B has a structural role in plants and that its ability to cross link pectin is a major factor in controlling the physical and biochemical properties of the primary wall. |
|
Arabidopsis mutants with altered RG-II structure and boron utilization |
|
This led us to believe that the dwarf phenotype of mur1 plants was a result of reduced RG-II cross linking. Indeed, spraying mur1 plants with aqueous borate restores the growth and also increases RG-II cross linking to near normal levels. The growth of mur1 plants is also restored by spraying with L-Fuc. These plants synthesize RG-II that contains L-Fuc and 2-O-methyl L-Fuc. Thus, RG-II dimer formation and stability and the B requirement of the L-Fuc-treated mur1 plants are similar to wild-type plants. |
 |
| Plants carrying the bor1 mutation are extremely dwarfed (9). The altered gene in these plants has been shown to encode a membrane-localized
protein that mediates the export of boron from pericycle cells into the
root stelar apoplasm. BOR1 increases the concentrations of B in the xylem
sap and thereby protects the aerial portions of the plant from B deficiency
(14). Wild-type and bor1 RG-II have similar glycosyl residue compositions. However, only 40% of the RG-II is cross-linked by borate in the mutant. Borate treatment rescues the growth of bor1 plants and restores RG-II cross-linking to normal levels. Thus, decreased shoot B is likely to lead to reduced cross linking of RG-II which itself may be repsonsible for the dwarf phenotype of the bor1 mutant. However, the possibility that B functions in a as yet unidentifed manner cannot be discounted. |
 |
| Borate cross-linked RG-II has been shown to account for <50% of the RG-II in nolac-H18 (Non-Organogenic callus with Lossely Attached Cells) generated by T-DNA transformation of haploid Nicotiana plumbaginifolia leaf discs (13). This callus has lost the ability to form tight intercellular attachments and adventituous roots. The affected gene (NpGUT1) is believed to encode for a RG-II-specific glucuronosyltransferase since the nolac-H18 RG-II lacks GlcA and is deficient in Gal (15). |
The cumulative results of several studies suggest that a primary function of boron is to covalently cross link wall pectin and that this cross linking is required for normal turgor-driven wall expansion to occur. There is no evidence to suggest that boron has a direct role in cell divsion. Nevertheless, much additional research is required to obtain a full understanding of the role of boron in primary walls and in plant growth and development. |
|
RG-II is a major polysaccharide component of red wine Most people are suprised to discover that 1 liter of red wine may contain between 100 and 150mg of RG-II. White wine typically contains 20 - 30 mg of RG-II per liter. These differences result form the different processe used to make red and white wines. White wines are made by fermenting grape juice which contain little of the cell wall. In contrast, red wines are made by fermenting grape berry pulp. The cell wall accounts for a large portion of the pulp and the RG-II is solubilized during the fermentation process. RG-II is extremely resistant to known microbial polysaccharide degrading enzymes and thus is not utilized as a carbon source during fermentation.
|
 |
|
1. Characterization of RG-II synthesized by Arabidopsis mutants whose
cell walls are deficient in arabinose (mur4) and rhamnose (mur8). |
| Selected references 1. Darvill et al (1978) Structure of plant cell walls. VIII. A new pectic polysaccharide. Plant Physiol., 62, 418-422. 2. Vidal et al. (2000) Structural characterization of the pectic polysaccharide rhamnogalacturonan II: evidence for the backbone location of the aceric acid-containing oligoglycosyl side chain. Carbohydr. Res., 326, 277-294. 3. Glushka et al (2003) Primary structure of the 2-O-methyl α-L-fucose-containing side chain of the pectic polysaccharide, rhamnogalacturonan II. Carbohydr. Res., 338, 341-352. 4. Kobayashi et al (1996) Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol., 119, 199-203. 5. ONeill et al (1996) Rhamnogalacturonan II, a pectic polysaccharide in the walls of growing plant cells, forms a dimer that is cross-linked by a borate ester - in vitro conditions for the formation and hydrolysis of the dimer. J Biol Chem., 271, 22923-22930. 6. Ishii et al (1999) The palnt cell wal polysaccharide rhamnogalacturonan II self-assembles into a covalently cross-linked dimer. J. Biol. Chem., 274, 13098-13109. 7. Ishii and Ono (1999) NMR spectroscopic analysis of the borate diol esters of methyl apiofuranosides. Carbohydr. Res., 321, 257-260. 8. Hu et al (1996) Species variability in boron requirement is correlated with cell wall pectin. J Exp Bot. 47, 227-232. 9. Noguchi et al (1997) bor1-1, an Arabidopsis thaliana mutant that requires a high level of boron. Plant Physiol, 115, 901-906. 10. Pellerin and O'Neill (1998) The interaction of the pectic polysaccharide rhamnogalacturonan II with heavy metals and lanthanides in wines and fruit juices. Analusis 26, M32-M36. 11. Kobayashi et al (1999) Borate-rhamnogalacturonan II bonding reinforced by Ca2+ retains pectic polysaccharides in higher plant cell walls. Plant Physiol., 119, 199-203. 12. Fleischer et al (1999) The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol., 121, 829-838. 13. ONeill et al (2001) Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science, 294, 846-849. 14. Takano et al (2002) Arabidopsis boron transporter for xylem loading. Nature, 420, 337-340. 15. Iwai et al (2002) A pectin glucuronosyltransferase gene is essential for intercellular attachment in the plant meristem. Proc. Nat. Acad. Sci. USA., 99, 16319-16324. 16. Matsunaga et al (2004) Occurence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes, lycophytes, and bryophytes. Implications for the evolution of vascular plants. Plant Physiol., 134, 339-351. |